Difference between revisions of "Projects:Utah2ShapeRegression"
Jfishbaugh (talk | contribs) |
Jfishbaugh (talk | contribs) |
||
| Line 13: | Line 13: | ||
[[File:Shape_regression_illustration.png|600px|center|thumb|Shape regression involves estimating a continuous evolution from a set of discrete shapes.]] | [[File:Shape_regression_illustration.png|600px|center|thumb|Shape regression involves estimating a continuous evolution from a set of discrete shapes.]] | ||
| − | |||
== Results == | == Results == | ||
| Line 22: | Line 21: | ||
geodesic evolution.]] | geodesic evolution.]] | ||
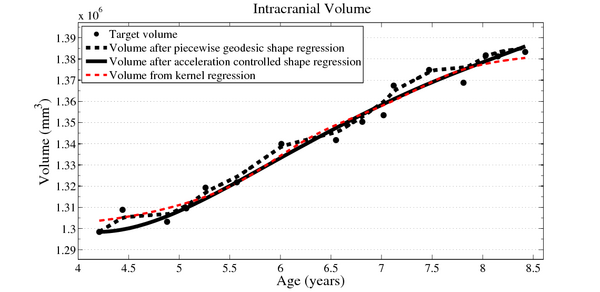
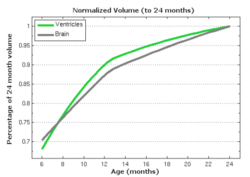
| − | [[File:Long_accel_volume_w_kernel_reg.png| | + | [[File:Long_accel_volume_w_kernel_reg.png|600px|center|thumb|olume measurements derived from our growth model are consistent with a kernel regression performed on the sparse volume measurements. Our model describes the continuous evolution of ''shape'' and volume is measured after regression.]] |
= Former Work = | = Former Work = | ||
Revision as of 00:11, 29 September 2011
Home < Projects:Utah2ShapeRegressionBack to Utah 2 Algorithms
Ongoing Work
Smooth Growth Trajectories from Time Series Shape Data
Segmentation of multi-time point image data of individual subjects results in serial shape data of structures of interest. Clinical research is interested in the spatiotemporal analysis of shape changes, which potentially leads to improved understanding of the rate of change, locality and growth trajectory of structures of interest.
Description
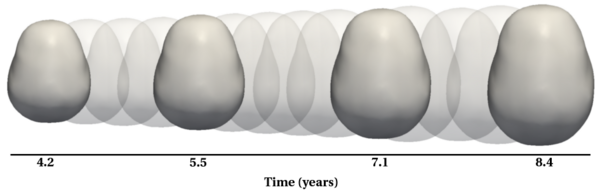
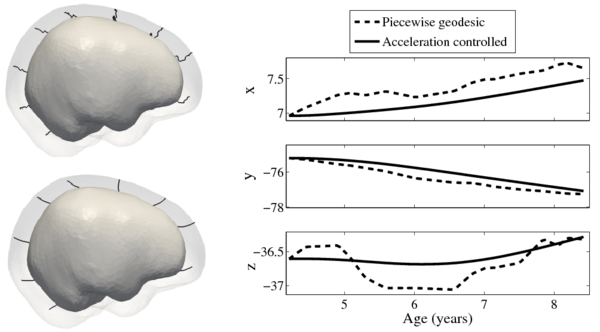
Longitudinal shape analysis often relies on the estimation of a realistic continuous growth scenario from data sparsely distributed in time. In this project, we develop a new type of growth model parameterized by acceleration, whereas standard methods usually control the velocity [1,2]. From this parameterization, we gain one order of differentiability and guarantee that shape evolution is smooth in both space and time. This mimics the behavior of biological tissue as a mechanical system driven by external forces. The growth trajectories are estimated as continuous flows of deformations, which are twice differentiable. The shape regression is based on a correspondence-free technique using currents [2,3], which calculates diffeomorphic flows between set of shapes represented as surface data.
Results
Former Work
In pilot tests on an infant growth study, we demonstrate that volume measurements taken out of our 3D shape regression are compatible with a 1D regression of these measurements. This implies that also other morphometric measurements of interest for clinicians might be smooth and thus model biological change in a realistic way. Leave-several-out experiments demonstrate that the new method better interpolates between data and is therefore more robust to missing observations. This suggests a greater ability to capture the underlying growth of the anatomical structures.
We plan to apply this method to anatomical shapes of image data on Huntington diseases (HD) provided the IOWA DBP partner. Preliminary studies showed an inflection of change rapid decrease of striatal volume trajectory, and application of this methodology to three time point data might reveal morphological characteristics of such volume loss.
Results
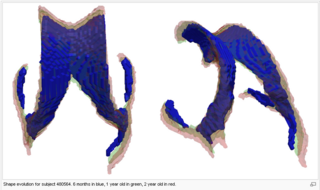
Tests of the prototype shape regression software on synthetic and real image data shows smooth shape evolution based on only few discrete time points. This property will be important when comparing shape change trajectories between different subjects, in particular if images/shapes are not taken at the same time. The regression is based on a correspondence-free method that uses the "currents" method for estimation of diffeomorphic registration. This property might be important when shapes with slightly different topology due to segmentation noise and biological change (see e.g. ventricles) are compared.
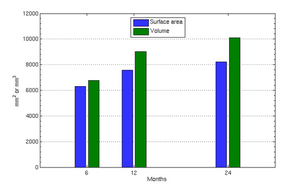
The following images show the example of brain ventricles and intracranial volumes (ICV) measured at 6 month, 1year and 2 years of age and the resulting smooth shape evolution.

Literature
[1] Miller, M, I., Trouve, A., Younes, L.: On the metrics and Euler-Lagrange equations of Computational Anatomy. Annual Review of Biomedical Engineering 4 (2002), 375–405
[2] Vaillant, M., Glaunes, J.: Surface matching via currents. In: Proceedings of IPMI. (2005) 381–392
[3] Durrleman, S., Pennec, X., Trouve, A., Gerig, G., Ayache, N.: Spatiotemporal atlas estimation for developmental delay detection in longitudinal datasets. In: Proc. of MICCAI’09, Part I. (2009) 297–304
Key Investigators
- Utah: James Fishbaugh, Stanley Durrleman, Guido Gerig
- IOWA: Hans Johnson