Difference between revisions of "Projects:TumorModeling"
| Line 24: | Line 24: | ||
= Key Investigators = | = Key Investigators = | ||
| − | * MIT: [http://people.csail.mit.edu/menze Bjoern Menze], Tammy Riklin Raviv, Koen Van Leemput, Polina Golland | + | * MIT: [http://people.csail.mit.edu/menze Bjoern Menze], [http://people.csail.mit.edu/tammy Tammy Riklin Raviv], [http://people.csail.mit.edu/koen Koen Van Leemput], [http://people.csail.mit.edu/polina Polina Golland] |
* INRIA Sophia-Antipolis, France: Ezequiel Geremia, Olivier Clatz, Nicholas Ayache | * INRIA Sophia-Antipolis, France: Ezequiel Geremia, Olivier Clatz, Nicholas Ayache | ||
* DKFZ Heidelberg, Germany: Bram Stieltjes, Marc-Andre Weber | * DKFZ Heidelberg, Germany: Bram Stieltjes, Marc-Andre Weber | ||
Revision as of 16:31, 13 March 2010
Home < Projects:TumorModelingBack to NA-MIC Collaborations, MIT Algorithms
Modeling tumor growth in patients with glioma
We are interested in developing computational methods for the assimilation of magnetic resonance image data into physiological models of glioma - the most frequent primary brain tumor - for a patient-adaptive modeling of tumor growth.
This aims at two directions: First, it aims at making complex information from longitudinal multimodal data set accessible for diagnostic radiology through physiological models. This will allow to estimate features such as degree of infiltration, speed of growth, or mass effect in a quantitative fashion; for therapy it will allow to identify regions at risk for progression. Second, it aims at providing the means to test different mavroscopic tumor models from theoretical biology on real clinical data.
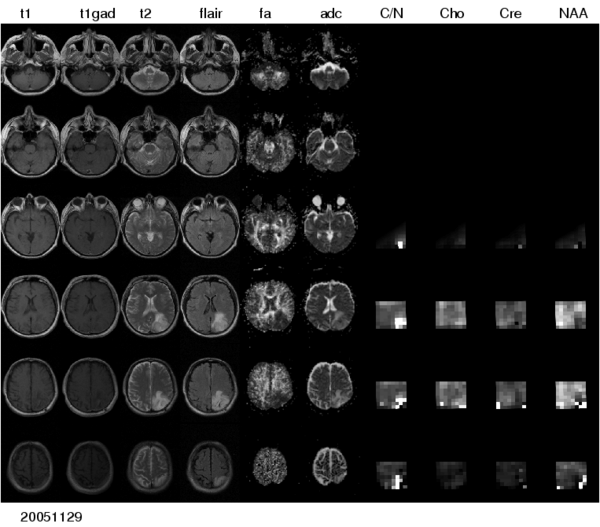
To realize these aims, the project comprises a number of ascpects -- automated segmentation of tumors in large multimodal image data sets, making information of different MR image modalities accessible for the tumor model, with a focus on the processing of magnetic resonance spectroscopic images (MRSI), and the development of methods for the image-based estimation of parameters in reaction-diffusion type models of tumor growth.
Segmenting tumors in large multimodal data sets
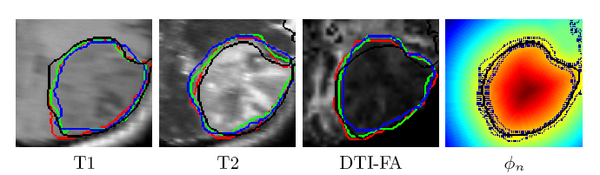
To segment all MR image volumes available for a patient we developed an approach for learning patient-specific lesion atlases (Figure 2) with limited user interaction. Figure 2 shows the manual segmentation of the tumor from different raters (red, green, blue) and the automatic segmentation using the patient-specific lesion atlas (black) in T1-MRI, T1-MRI and the fractional anisotropy map from DTI.
Processing magnetic resonance spectroscopic images
To make the metabolic information of magnetic resonance spectroscopic images available for modeling the evolution of glioma growth we are implementing an MRSI processing module for Slicer.
Key Investigators
- MIT: Bjoern Menze, Tammy Riklin Raviv, Koen Van Leemput, Polina Golland
- INRIA Sophia-Antipolis, France: Ezequiel Geremia, Olivier Clatz, Nicholas Ayache
- DKFZ Heidelberg, Germany: Bram Stieltjes, Marc-Andre Weber