Projects:TumorModeling
Back to NA-MIC Collaborations, MIT Algorithms
Modeling tumor growth in patients with glioma
We are interested in developing computational methods for the assimilation of magnetic resonance image data into physiological models of glioma - the most frequent primary brain tumor - for a patient-adaptive modeling of tumor growth.
This aims at two directions: First, it aims at making complex information from longitudinal multimodal data set accessible for diagnostic radiology through physiological models. This will allow to estimate features such as degree of infiltration, speed of growth, or mass effect in a quantitative fashion; for therapy it will allow to identify regions at risk for progression. Second, it aims at providing the means to test different macroscopic tumor models from theoretical biology on real clinical data.
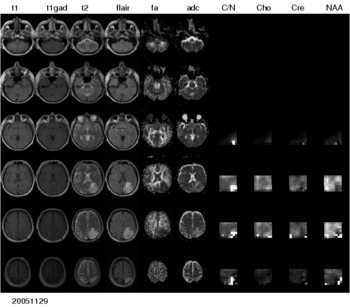
To realize these aims, the project comprises a number of aspects -- automated segmentation of tumors in large multimodal image data sets, making information of different MR image modalities accessible for the tumor model, with a focus on the processing of magnetic resonance spectroscopic images (MRSI), and the development of methods for the image-based estimation of parameters in reaction-diffusion type models of tumor growth.
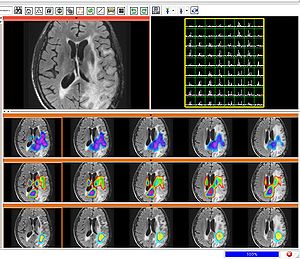
Segmenting tumors in large multimodal data sets
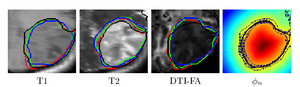
To segment all MR image volumes available for a patient we developed an approach for learning patient-specific lesion atlases (Figure 2) with limited user interaction. Figure 2 shows the manual segmentation of the tumor from different raters (red, green, blue) and the automatic segmentation using the patient-specific lesion atlas (black) in T1-MRI, T1-MRI and the fractional anisotropy map from DTI.
Reference: Menze BH, Van Leemput K, Lashkari D, Weber MA, Ayache N and Golland P. A generative model for brain tumor segmentation in multi-modal images. Proc MICCAI 2010. LNCS 6362, 151-59
Image-based modeling of tumor growth
We propose a joint generative model of tumor growth and of image observation that naturally handles multi-modal and longitudinal data. We use the model for analyzing imaging data in patients with glioma. The tumor growth model is based on a Fisher-Kolmogorov reaction-diffusion framework. Model personalization relies only on a forward model for the growth process and on image likelihood. We take advantage of an adaptive sparse grid approximation for efficient inference via Markov Chain Monte Carlo sampling. The approach can be used for integrating information from different multi-modal imaging protocols and can easily be adapted to other tumor growth models.
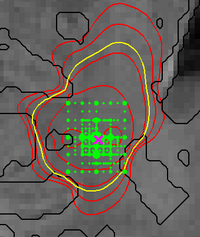
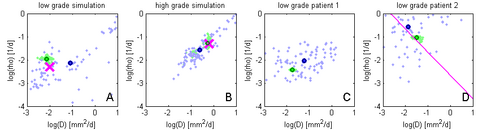
Figure 4 illustrates the adaptive grid sampling of the parameter space for a modeled tumor. The size of the green sampling points indicates how often the indicated location parameter of the tumor model was evaluated under different parametrizations. The ground truth is indicated by the pink cross. Most adaptively chosen sampling points are close to the ground truth. The figure also shows isolines of tumor cell density (red), the predicted extensions of the T2 hyper-intense area (yellow) and tissue boundaries (black).
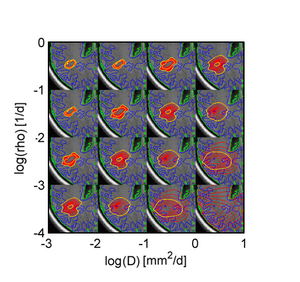
Figure 5 shows results of the proposed approach: Green samples are obtained from the proposed sparse grid approach while blue sample are obtaine via standard MCMC. Black circles indicate means of the two distributions. Ground truth for A and B are indicated by the pink cross. In D the previously estimated speed of growth [7] is shown by the pink line. The sparse grid sampling approximation performs better than the direct MCMC (A-B). Estimates correlate well with privously published results, but provide a more accurate characterization of the state of disease (D).
Reference: Menze BH, Van Leemput K, Honkela A, Konukoglu E, Weber MA, Ayache N and Golland P. A generative approach for image-based modeling of tumor growth. Proc IPMI 2011. LNCS.
Processing magnetic resonance spectroscopic images
To make the metabolic information of magnetic resonance spectroscopic images available for modeling the evolution of glioma growth we are implementing an MRSI processing module for Slicer.
Key Investigators
- MIT: Bjoern Menze, Tammy Riklin Raviv, Koen Van Leemput, Polina Golland
- Harvard: William M. Wells
- INRIA Sophia-Antipolis, France: Ezequiel Geremia, Olivier Clatz, Nicholas Ayache
- DKFZ Heidelberg, Germany: Bram Stieltjes, Marc-Andre Weber