DBP:TBI
TRAUMATIC BRAIN INJURY
PI: Jack Van Horn, UCLA
In vivo neuroimaging is an increasingly relevant means for the neurological assessment of traumatic brain injury (TBI) [1]. However, standard automated image analysis methods are not sufficiently robust with respect to TBI-related changes in image contrast, brain shape, cranial fractures, white matter fiber alterations, and other signatures of head injury. Associating multimodal quantification of brain insults with clinical and outcome metrics is a particular challenge. This DBP employs a multi-modal approach coupled with clinical outcome measures to build computational models for guided and semi-automatic TBI analysis and quantification with a view toward assessing clinical improvement under the following Specific Aims.
Specific Aims
1. Develop end-to-end processing approaches using the NA-MIC Kit to investigate alterations in cortical thickness, and subsequent ventricular and white matter changes in patients with TBI and in age-matched controls. Image processing will include segmentation of lesions, hemorrhage, edema, and other pathology relevant to TBI. Longitudinal changes will be assessed by registration and joint segmentation of baseline and follow-up data.
2. Develop robust workflows for diffusion weighted imaging (e.g. DTI, HARDI) datasets from TBI patients, by using the NA-MIC Kit and Slicer to obtain reliable and robust metrics of white matter pathology and of white matter changes due to therapy and/or recovery.
3. Using the NA-MIC Kit, cross-correlate multimodal metrics of cortical thickness, complexity, ventricular volume, and lesions from structural imaging and white matter fiber integrity from diffusion tensor imaging, with clinical outcome variables, i.e., time since injury, age, gender and other potential factors predictive of recovery (Figure 1). Emphasis is placed on the feasibility of subject-specific analysis, as opposed to population-based averaging, to examine the influence of TBI on time-dependent alteration of gray and white matter integrity with accompanying change in clinical outcome variables to be used in subsequent TBI assessment.
Background
Traumatic Brain Injury, also called diffuse axonal injury (DAI), acquired brain injury or, simply, head injury, occurs when a sudden trauma causes damage to the brain [2]. TBI results when the head suddenly and violently hits an object, or when an object pierces the skull and enters brain tissue. Symptoms can be mild, moderate, or severe, depending on the extent of the damage to the brain [3]. Common disabilities include deficits in cognition, sensory processing, communication, and behavior or mental health [4-6]. Severe TBI may result in stupor, individual can be aroused briefly by a strong stimulus (e.g., sharp pain); coma, individual is totally unconscious, unresponsive, unaware, and unarousable; vegetative state, individual is unconscious and unaware of his or her surroundings, but continues to have a sleep-wake cycle and periods of alertness; and persistent vegetative state, individual remains unresponsive for more than a month [7].
According to the Centers for Disease Control and Prevention [8], TBI affects 1.4 million Americans annually resulting in $60 billion in healthcare costs (in year 2000). Of those affected yearly, 50,000 die, 235,000 are hospitalized for some length of time, and 1.1 million are treated and released from a hospital emergency department. The CDC also estimates that at least 5.3 million Americans currently have a long-term or lifelong need for help to perform activities of daily living as a result of TBI. Few, if any, follow-up treatment options exist. Accordingly, ~40% of those hospitalized with TBI have at least one unmet need for services 1-year post-injury. These unmet needs include improving memory and problem-solving; managing stress and emotional upsets; and improving one’s job skills. The most common demographic for TBI is 16-24 y.o. males, who are affected at a critical time of learning and formal entry into the U.S. workforce [7]. Principal among this demographic are returning Iraq and Afghanistan war veterans [9]. The long-term effects of TBI include epilepsy and increased risk for diseases such as Alzheimer’s [10], Parkinson’s [11], and other brain disorders that become more prevalent with age [12]. The magnitude of this medical concern to the U.S. cannot be over-stated.
Neurological Concomitants of TBI. Following TBI, a cascade of neuroanatomical alterations initiate, with diffuse alterations in cortical structure peripheral to the point of injury but also distributed throughout the brain [6]. Notably, there is ventricular enlargement and cortical thickness changes remote from the site of the TBI. White matter connectivity can be significantly altered with greatly reduced efficiency of signal transduction over affected pathways or complete cessation of inter-regional communication due to axonal damage. These anatomical derangements can have profound effects on speech, motor, and cognitive processes [5, 13]. The extent of change is putatively related to the severity of TBI, location, subject age, and post-injury reatment, among other factors.
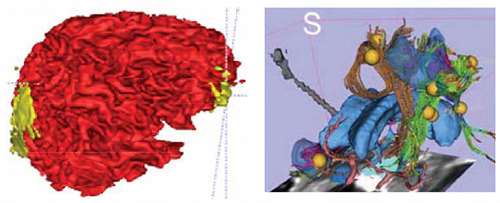
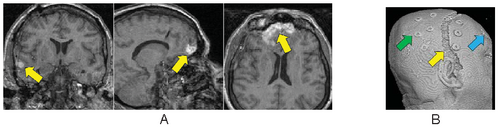
Computational and Data Processing Issues. On anatomical MRI scans, TBI-related insults can appear as hyper-intensities (Figure 1A), which vary in magnitude and extent, the degree to which tends to correlate with clinical symptoms [14]. Additionally, in severe TBI, sections of skull that are fractured during the injury or removed during surgical intervention (Figure 1B) may not form a contiguous boundary to enable efficient digital removal of bone and other non-brain tissues. This, in turn, complicates tissue segmentation, regional parcellation, the measurement of ventricular size, cortical thickness, and other metrics [15]. Computational algorithms require refinement to include constraints to account for TBI-related signal alterations in anatomical scans; e.g., users may have to manually indicate regions encompassing the site of injury on the scans to guide local processing around the site and to reduce the weight of these regions on other, nonaffected brain areas. Alternatively, probabilistic classifiers may need to include an extra classification for voxels with tissue properties that have been altered by TBI (Figure 2). This is particularly the case in diffusion weighted imaging (DWI) where the presence of TBI-related alterations in signal may reflect specific damage to white matter proximal to the lesion as well as long reaching effects along tracts to peripherally connected regions of cortex [3,16,17].
Use of NA-MIC Resources and the NA-MIC Kit. Using sophisticated NA-MIC tools, this DBP is developing end-to-end processing solutions by which to examine TBI neuroimaging data. The NA-MIC Kit encompasses a collection of tools for automated or semi-automated processing of medical imaging data. Notable among these is the Insight Toolkit [18] for use in brain registration and segmentation via the 3D Slicer software platform [19]. These software tools may be linked to form data processing workflows that can process data via end-to-end solutions that may be shared with others, posted on websites, and used in training materials. They form an excellent platform for user-guided, patient-specific analysis, but require additional development to inform the program about regions where TBI-related signal changes may necessitate alteration of model parameters or search volumes.
Investigators
| NAME | DEGREE | INSTITUTION | EXPERIENCE | ROLE |
| JACK VAN HORN | PH.D. | UCLA | NEUROSCIENCE & ENGINEERING | DBP PI |
| DAVID HOVDA | PH.D. | UCLA | NEUROSURGICAL OUTCOMES | INVESTIGATOR |
| PAUL VESPA | M.D. | UCLA | NEUROINTENSIVIST | INVESTIGATOR |
| ARTHUR TOGA | PH.D. | UCLA | NEUROSCIENCE & INFORMATICS | INVESTIGATOR |
| JEFFREY ALGER | PH.D. | UCLA | NEUROIMAGING | INVESTIGATOR |
| GUIDO GERIG | PH.D. | UTAH | COMPUTER SCIENCE | LEAD TEDHNICAL CONTACT |
| STEPHEN AYLWARD | PH.D. | UNC | ENGINEERING | LEAD TECHNICAL CONTACT |
Methods
Analysis Software Tools and Data Processing Protocol. Specifically adopt the NA-MIC Kit open source software platform consisting of Slicer, tools and toolkits such as VTK and ITK, and software engineering methodologies for multiplatform implementation. Using ITK, data will be intensity normalized and bias-field corrected; tissue types will be segmented interactively to assist probabilistic classification; cortical thickness will be determined along the entire cortical sheet as the linear distance between the outer edge of the cortical surface and the grey-white matter boundary. Ventricular size will be determined by a space filling algorithm, while shape will be characterized using LONI tools for shape decomposition and quantitative description. DWI processing routines will be developed to better account for TBI-related changes in diffusion metrics. Results from multimodal analyses will be visualized using VTK, Slicer, and other suitable platforms.
Exemplar Anatomical Data. Examine neuroimaging data obtained from TBI patients to rigorously assess workflows using the NA-MIC Kit. MRI volumes from 202 subjects will be drawn from the LONI Image Data Archive (IDA), a comprehensive neuroimaging data archive comprised of a number of funded projects. Samples will include patients who have suffered from TBI (N=160; 22F, 138M) and age-matched normal controls (N=42; 13F, 29M). Mean ages for males is 33.8±9.2 and for females is 33.6±9.9. T1-weighted whole brain MPRAGE volumes, T2, and, in subjects with available data sets, diffusion weighted imaging (DTI/HARDI) collected at 1.5 and 3.0T will be utilized. Additional data include a variety of MR imaging modalities and NAMIC workflows will be crafted to accommodate them.
Data Analysis and Expected Results. Obtain multimodal results using Slicer software tools, specifically developed under NA-MIC using ITK, VTK, for the analysis of neurological concomitants of TBI. Metrics will be extracted and imported into purpose-built software for univariate and multivariate modeling to provide additional insights to that of previous work on the role of neuroanatomical changes occurring in TBI on outcome variables predicting degree of change and/or recovery. Several primary hypotheses using individual and repeated imaging include: (1) Cerebral atrophy (regional and global) occurs at a faster rate in diffuse vs. focal TBI; (2) Rates are dependent upon initial injury severity; (3) Ongoing or progressive change continues up to 1 year post-TBI; and (4) Secondary insults increase the rate and extent of the initial TBI. We will also examine age-at-lesion effects, since these factors are likely to impact on measures of the degree of loss of developmental and life-span neuroplasticity believed to follow TBI. Using DWI data, we will assess the effects of TBI on mean diffusivity, fractional anisotropy, and their potential as clinical outcome correlates. Complete multimodal data processing solutions using the NA-MIC Kit and associated tools will be made openly available, with accompanying training materials via the NA-MIC web site, and comply with the NA-MIC open-source policies.
Clinical Utility. The NA-MIC Kit workflows developed under this program are intended for application to TBI clinical practice and patient monitoring. We do not envision the tools being used to pool brain image data across subjects (e.g. the creation of brain atlases, per se) but for use in assessing extent of traumatic brain damage and measuring change over time in individual subjects. However, with knowledge of general location, extent, and degree of change, such metrics can be associated with clinical measures and used to suggest viable treatment options for a subject against patterns typical of TBI patients.
Connections between this work and any of the other 3 proposed DBPs
Considerable interactions presently exist between the NA-MIC project and the Laboratory of Neuro Imaging (LONI), and have resulted in several collaborative peer-reviewed publications, underscoring the suitability of this collaboration. Work under this DBP involves direct interaction with the Engineering team, as well as the Head and Neck Cancer DBP project being conducted at MGH.
Deliverables, Timeline, Impact
| SPECIFIC AIMS | Year 1 | Year 2 | Year 3 |
| Aim 1 | Design and evaluate end-to-end processing approaches for segmentation of multimodal images Cortical thickness, segmentation of ventricles, lesions, bleedings, hemorrhage | Evaluate registration of initial and followup scans, extend segmentation processing to joint segmentation of longitudinal image data, development of workflows, documentation | Develop framework for assessment of longitudinal changes of altered brain anatomy and pathology w.r.t.. brain plasticity and secondary neuroanatomical effects |
| Aim 2 | Test existing DWI processing modules, design of refined tools for analysis of white matter pathology in TBI | Develop workflows for efficient extraction and analysis of DWI data from TBI patients, extension to serial data of individual patients | Use workflows and 3D Slicer visualizations to assess white matter changes due to therapy and/or recovery, documentation |
| Aim 3 | Develop schemes descriptive for TBI for specification of metrics from segmentations of gray and white matter brain alterations and pathology | Establishing image-derived multimodal metrics of gray and white matter pathology in TBI, extends metrics by including longitudinal changes | Cross correlation of multimodal metrics with clinical outcome variables to evaluate potential factors that are predictive of recovery and can inform and guide clinical assessment |
References
- Furlow B. Diagnostic imaging of traumatic brain injury. Radiol Technol. 2006;78(2):145-56; quiz 57-9.
- Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503-57. PMCID: 1462953.
- Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22(2):115-22. PMID: 18240040.
- Fleminger S. Long-term psychiatric disorders after traumatic brain injury. Eur J Anaesthesiol Suppl. 2008;42:123-30. PMID: 18289429.
- McDonald BC, Flashman LA, Saykin AJ. Executive dysfunction following traumatic brain injury: neural substrates and treatment strategies. NeuroRehabilitation. 2002;17(4):334.
- Satz P, Zaucha K, Forney DL, McCleary C, Asarnow RF, Light R, et al. Neuropsychological, psychosocial and vocational correlates of the Glasgow Outcome Scale at 6 months post-injury: a study of moderate to severe traumatic brain injury patients. Brain Inj. 1998;12(7):555-67. PMID: 9653519.
- Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728-41. PMID: 18635021.
- CDC (http://www.cdc.gov/NCIPC/tbi/Fact-Sheets/Facts_About_TBI.pdf).
- Connors S, Gordon WA, Hovda DA. Care of war veterans with mild traumatic brain injury. N Engl J Med. 2009;361(5):536-7; author reply 7-8. PMID: 19645084.
- Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. Prog Brain Res. 2007;161:303-16. PMID: 17618986.
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326-37. PMID: 176261678.
- Tupler LA, Krishnan KR, McDonald WM, Dombeck CB, D’Souza S, Steffens DC. Anatomic location and laterality of MRI signal hyperintensities in late-life depression. J Psychosom Res. 2002;53(2):665-76. PMID: 12169341.
- Ruttan L, Martin K, Liu A, Colella B, Green RE. Long-term cognitive outcome in moderate to severe traumatic brain injury: a meta-analysis examining timed and untimed tests at 1 and 4.5 or more years after injury. Arch Phys Med Rehabil. 2008;89(12 Suppl):S69-76.
- Dubroff JG, Newberg A. Neuroimaging of traumatic brain injury. Semin Neurol. 2008;28(4):548-57. PMID: 18843581
- Poca MA, Sahuquillo J, Mataro M, Benejam B, Arikan F, Baguena M. Ventricular enlargement after moderate or severe head injury: a frequent and neglected problem. J Neurotrauma. 2005;22(11):1303-10. PMID: 16305318.
- Kumar R, Husain M, Gupta RK, Hasan KM, Haris M, Agarwal AK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neurocognitive function. J Neurotrauma. 2009;26(4):481-95. PMID: 19196176.
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29(5):967-73. PMID: 18272556.
- Pieper S., Lorensen B., Schroeder W., Kikinis R. The NA-MIC Kit: ITK, VTK, Pipelines, Grids and 3D Slicer as an Open Platform for the Medical Image Computing Community. Proceedings of the 3rd IEEE International Symposium on Biomedical Imaging: From Nano to Macro 2006; 1:698-701.
- Pieper S., Halle M., Kikinis R. 3D SLICER. Proceedings of the 1st IEEE International Symposium on Biomedical Imaging: From Nano to Macro 2004; 1:632-635.
- Van Horn JD, Toga AW. Multisite neuroimaging trials. Curr Opin Neurol. 2009;22(4):370-8. PMCID: 19506479.
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 007;130(Pt10):2508-19. PMID: 17872928.