Difference between revisions of "DPB2:Past Featured Articles"
From NAMIC Wiki
(New page: Back to NA-MIC DBP 2 * <div id="fichtinger_miccai_07">'''G. Fichtinger et al. Robotic Assistance for Ultrasound Guided Prostate Brachytherapy...) |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
{| | {| | ||
| − | | | + | | width="20%" align="left" valign="top" | |
| − | [[Image:ProstateDiagram.png| | + | [[Image:ProstateDiagram.png|left|200px]] |
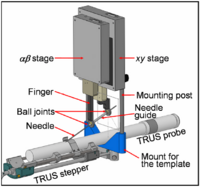
| − | | valign="top" | We present a robotically assisted prostate brachytherapy system and test results in training phantoms. The system consists of a transrectal ultrasound (TRUS) and a spatially co-registered robot integrated with an FDA-approved commercial treatment planning system. The salient feature of the system is a small parallel robot affixed to the mounting posts of the template. The robot replaces the template interchangeably | + | | align="left" valign="top" |We present a robotically assisted prostate brachytherapy system and test results in training phantoms and Phase-I clinical trials. The system consists of a transrectal ultrasound (TRUS) and a spatially co-registered robot, fully integrated with an FDA-approved commercial treatment planning system. The salient feature of the system is a small parallel robot affixed to the mounting posts of the template. The robot replaces the template interchangeably, using the same coordinate system. Established clinical hardware, workflow and calibration remain intact. In all phantom experiments, we recorded the first insertion attempt without adjustment. All clinically relevant locations in the prostate were reached. Non-parallel needle trajectories were achieved. The pre-insertion transverse and rotational errors (measured with a Polaris optical tracker relative to the template's coordinate frame) were 0.25 mm (STD=0.17 mm) and 0.75 deg (STD=0.37 deg). In phantoms, needle tip placement errors measured in TRUS were 1.04 mm (STD=0.50 mm). A Phase-I clinical feasibility and safety trial has been successfully completed with the system. We encountered needle tip positioning errors of a magnitude greater than 4 mm in only 2 out of 179 robotically guided needles, in contrast to manual template guidance where errors of this magnitude are much more common. Further clinical trials are necessary to determine whether apparent benefits of the robotic assistant will lead to improvements in clinical efficacy and outcomes. |
|- | |- | ||
| − | | | + | || |
| − | Gabor Fichtinger, Jonathan Fiene, Christopher W. Kennedy, Gernot Kronreif, Iulian I. Iordachita, Danny Y. Song, E. Clif Burdette, and Peter Kazanzides | + | |align="left"|Gabor Fichtinger, Jonathan Fiene, Christopher W. Kennedy, Gernot Kronreif, Iulian I. Iordachita, Danny Y. Song, E. Clif Burdette, and Peter Kazanzides. [[Media:Gabor-robotic07.pdf| Robotic Assistance for Ultrasound Guided Prostate Brachytherapy]]. Med Image Anal. 2008 Oct;12(5):535-45. |
|} | |} | ||
Latest revision as of 15:06, 14 September 2010
Home < DPB2:Past Featured ArticlesBack to NA-MIC DBP 2
- G. Fichtinger et al. Robotic Assistance for Ultrasound Guided Prostate Brachytherapy
| We present a robotically assisted prostate brachytherapy system and test results in training phantoms and Phase-I clinical trials. The system consists of a transrectal ultrasound (TRUS) and a spatially co-registered robot, fully integrated with an FDA-approved commercial treatment planning system. The salient feature of the system is a small parallel robot affixed to the mounting posts of the template. The robot replaces the template interchangeably, using the same coordinate system. Established clinical hardware, workflow and calibration remain intact. In all phantom experiments, we recorded the first insertion attempt without adjustment. All clinically relevant locations in the prostate were reached. Non-parallel needle trajectories were achieved. The pre-insertion transverse and rotational errors (measured with a Polaris optical tracker relative to the template's coordinate frame) were 0.25 mm (STD=0.17 mm) and 0.75 deg (STD=0.37 deg). In phantoms, needle tip placement errors measured in TRUS were 1.04 mm (STD=0.50 mm). A Phase-I clinical feasibility and safety trial has been successfully completed with the system. We encountered needle tip positioning errors of a magnitude greater than 4 mm in only 2 out of 179 robotically guided needles, in contrast to manual template guidance where errors of this magnitude are much more common. Further clinical trials are necessary to determine whether apparent benefits of the robotic assistant will lead to improvements in clinical efficacy and outcomes. | |
| Gabor Fichtinger, Jonathan Fiene, Christopher W. Kennedy, Gernot Kronreif, Iulian I. Iordachita, Danny Y. Song, E. Clif Burdette, and Peter Kazanzides. Robotic Assistance for Ultrasound Guided Prostate Brachytherapy. Med Image Anal. 2008 Oct;12(5):535-45. |