Difference between revisions of "MeningiomaMRIRegistrationStudy"
From NAMIC Wiki
(→Data) |
|||
| Line 15: | Line 15: | ||
* Expert landmarks for registration evaluation: not available | * Expert landmarks for registration evaluation: not available | ||
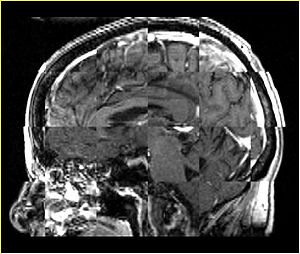
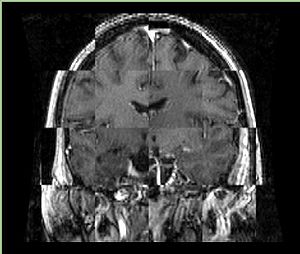
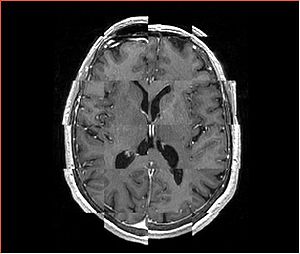
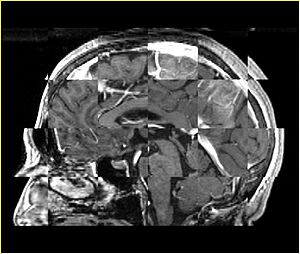
* Checkerboard appearance of unregistered images for the representative data of interest | * Checkerboard appearance of unregistered images for the representative data of interest | ||
| + | |||
| + | ** Case01 | ||
| + | <gallery perrow="3" heights="300px" widths="300px"> | ||
| + | Image:Case01-axial.jpg | ||
| + | Image:Case01-saggital.jpg | ||
| + | Image:Case01-coronal.jpg | ||
| + | </gallery> | ||
| + | |||
| + | ** Case03 | ||
| + | <gallery perrow="3" heights="300px" widths="300px"> | ||
| + | Image:Case03-axial.jpg | ||
| + | Image:Case03-saggital.jpg | ||
| + | Image:Case03-coronal.jpg | ||
| + | </gallery> | ||
| + | |||
| + | ** Case04 | ||
| + | <gallery perrow="3" heights="300px" widths="300px"> | ||
| + | Image:Case04-axial.jpg | ||
| + | Image:Case04-saggital.jpg | ||
| + | Image:Case04-coronal.jpg | ||
| + | </gallery> | ||
** Case10 | ** Case10 | ||
| − | <gallery | + | <gallery perrow="3" heights="300px" widths="300px"> |
Image:Case10-axial.jpg | Image:Case10-axial.jpg | ||
Image:Case10-saggital.jpg | Image:Case10-saggital.jpg | ||
| Line 27: | Line 48: | ||
* qualitative assessment: visually pleasing results | * qualitative assessment: visually pleasing results | ||
* quantitative assessment: something better than "visually pleasing" (TBD) | * quantitative assessment: something better than "visually pleasing" (TBD) | ||
| − | * minimum execution time | + | * minimum execution time to meet the application requirements: interactive quantification of tumor growth |
=Methods= | =Methods= | ||
Revision as of 15:50, 20 July 2009
Home < MeningiomaMRIRegistrationStudyContents
Objective
Accurate registration of same patient/same modality MRI data for longitudinal analysis of tumor progression.
Specific Aims
- Compare the accuracy of registration using existing Slicer and non-Slicer tools
- Identify parameter settings that produce satisfactory results
- Outline the limitations of the available registration tools in the context of the specific clinical research application
Data
- Input images: isotropic post-contrast T1 MRI acquired at different locations of BWH during 2006-2008, used under medical records study IRB. Time period between acquisition of scans for each patient is about 1 year.
- Ground truth transformation: not available
- Expert landmarks for registration evaluation: not available
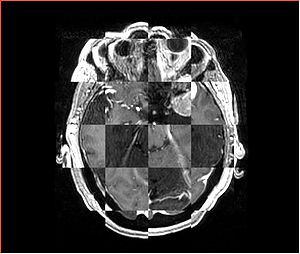
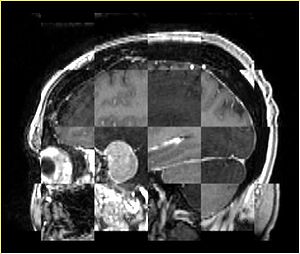
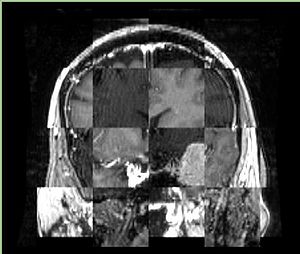
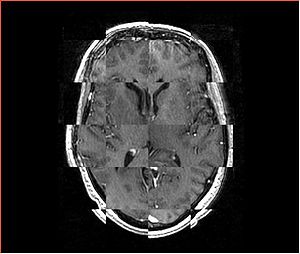
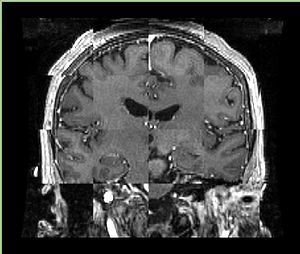
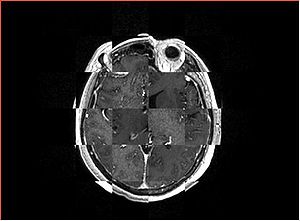
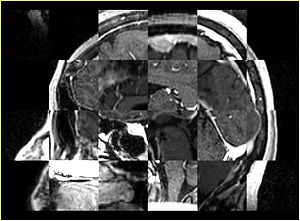
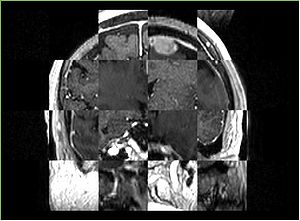
- Checkerboard appearance of unregistered images for the representative data of interest
- Case01
- Case03
- Case04
- Case10
Measures of success
- qualitative assessment: visually pleasing results
- quantitative assessment: something better than "visually pleasing" (TBD)
- minimum execution time to meet the application requirements: interactive quantification of tumor growth